February 2025 TPJ Editor choice: Phosphorylation in two acts: Marchantia phototropin undergoes sequential cis- and trans-autophosphorylation
Highlighted publication:
Phototropin switches between cis- and trans-autophosphorylation in light-induced chloroplast relocation in Marchantia polymorpha
https://doi.org/10.1111/tpj.17183
Phosphorylation in two acts: Marchantia phototropin undergoes sequential cis- and trans-autophosphorylation
Plants optimise photosynthetic performance and protect themselves from light-induced damage by sensing the intensity, periodicity, composition and direction of light. Consequently, plants have evolved several sets of photoreceptors to monitor their light environment. Phototropins are UV-A and blue light receptors that regulate responses such as phototropism, leaf flattening, chloroplast positioning and stomatal opening. Among these, light-induced chloroplast movement is one of the most conserved processes and can be observed not only in seed plants, but also in green algae, mosses and liverworts. Under low light conditions, chloroplasts accumulate at cell walls and orient perpendicular to the incoming light, maximising photosynthetic efficiency. Strong light intensities instead elicit an avoidance response where chloroplasts reorient parallel to the incoming light. This is generally thought of as a photoprotective mechanism, avoiding light-induced damage to the photosynthetic apparatus, but also allowing light to penetrate into deeper tissues.
Phototropins contain two light-sensing LOV (Light, Oxygen, or Voltage-sensing) domains and a C-terminal serine/threonine kinase domain. In darkness, the LOV2 domain represses the kinase activity. Upon light absorption, this repression is relieved, triggering autophosphorylation of the kinase domain at multiple serine and threonine residues – an essential step in phototropin signalling. Phototropins form dimers, suggesting that autophosphorylation can occur both in cis (by the molecule’s own kinase domain) and in trans (by its dimer partner). Arabidopsis phototropin 1 (Atphot1) was shown to undergo trans-autophosphorylation, but such mechanistic insight remains scarce for phototropins of other species.
The liverwort Marchantia polymorpha (Marchantia) harbours a single copy phototropin gene (MpPHOT) that mediates chloroplast accumulation and avoidance responses under weak and strong light, respectively, as well as avoidance responses at low temperatures. Noguchi et al. investigated the effects of these light and temperature conditions on Mpphot autophosphorylation. They observed that the Mpphot protein in extracts from blue-light grown plants appeared larger (i.e. exhibited slower mobility) on an immunoblot than protein from dark-grown plants, suggesting increased autophosphorylation. Interestingly, the level of phosphorylation differed with light intensity and temperature: while weak blue light caused a moderate mobility shift at 22 °C, both strong blue light at 22 °C and weak blue light at low temperatures of 5 °C resulted in a stronger mobility shift, suggesting a further increase in phosphorylation (hyperphosphorylation) (Fig. 1A).
Mass spectrometry analysis of purified, Citrine-tagged Mpphot (Mpphot-Cit) identified a total of 23 serine/threonine phosphosites, 21 of which were detected in both blue light- and dark-treated samples. Weak blue light resulted in an overall increase in the number of phosphopeptides at all 23 sites, with further increases observed under low temperature, suggesting that activation of Mpphot occurs via a global rise in serine/threonine phosphorylation across the entire protein, rather than via site-specific modifications.
Noguchi et al. then generated a kinase-inactive Mpphot variant (Mpphot-KI-Cit) by mutating a conserved aspartic acid residue in the kinase domain to prevent cis-autophosphorylation. When introduced into wild-type Marchantia plants, Mpphot-KI-Cit could only undergo trans-autophosphorylation by endogenous Mpphot and did not display a mobility shift under weak blue light, suggesting that primary autophosphorylation under these conditions occurs in cis. However, a mobility shift was detected for Mpphot-KI-Cit under strong blue light or at low temperature, suggesting that these conditions promote trans-autophosphorylation. Furthermore, a time course analysis during a shift from darkness to strong blue light showed that cis-autophosphorylation preceded trans-autophosphorylation.
Interestingly, Mpphot-KI-Cit exerted a dominant negative effect on endogenous Mpphot, suppressing Mpphot trans-autophosphorylation under strong blue light or at low temperature. This suppression also impaired Mpphot function: Mpphot-KI-Cit lines showed a wild type-like chloroplast accumulation response under weak blue light, but the avoidance responses were attenuated under strong blue light or at low temperature, suggesting that trans-autophosphorylation of endogenous Mpphot is critical for cold and high light acclimation in Marchantia.
Taken together, Noguchi et al. show for the first time that a phototropin undergoes both cis- and trans-autophosphorylation in a coordinated manner, providing novel insight into phototropin’s mode of action in Marchantia. This combination of cis- and trans-autophosphorylation allows for rapid signal amplification, which may increase the speed and efficiency of cellular responses to changing light environments.
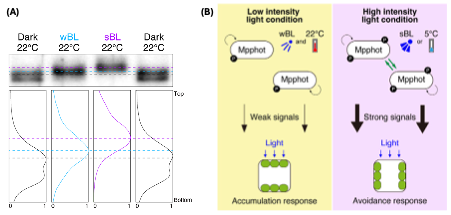
Figure 1. Control of Mpphot cis- and trans-autophosphorylation.
- Immunobot and band intensity histograms of Mpphot in protein extracts of Marchantia grown in dark, weak blue light (wBL) or strong blue light (sBL) conditions. Mpphot was detected with anti-MpPHOT antibodies.
- Model for the roles of cis- and trans-autophosphorylation of Mpphot in chloroplast positioning. Under wBL, only cis-autophosphorylation occurs and is sufficient to trigger the accumulation response. Both cis- and trans-autophosphorylation occur during avoidance responses under sBL or at low temperatures.
Figure modified from Noguchi et al. (2025).






