November 2024 TPJ Editor choice
Exit control: the role of Arabidopsis hydathodes in auxin storage and nutrient recovery
Highlighting: https://doi.org/10.1111/tpj.17014
Hydathodes are organs on the leaves of all vascular plants. They regulate the secretion of fluids derived from the xylem sap. When stomata are closed at night and the humidity level levels are too high, the xylem delivers excess water from the roots, which is secreted at the hydathodes in a process called guttation. Hydathodes are composed of an epidermal surface layer with water pores, and an inner parenchyma, called the epithem, which is highly vascularized and constitutes a direct connection between leaf surface and xylem vessels.
Routaboul et al. tested two long-standing hypotheses about hydathodes: that hydathodes are sites of auxin accumulation, and that they facilitate the withholding of nutrients from guttation fluids. For their study, Routaboul et al. combined RNAseq of hydathode-enriched tissue by deep sequencing with a detailed metabolomic analysis of guttation fluids.
First, the authors compared the transcriptome of macro-dissected leaf margins containing hydathodes with the transcriptome of leaf blade tissue of mature Arabidopsis leaves. They found higher expression of genes related to auxin metabolism, stress, DNA, plant cell wall, transport, RNA and lipids in the hydathode-enriched tissue. Alongside a higher expression of auxin biosynthesis, signalling and response genes in the hydathodes, there was more free auxin and more oxindole-3-AIA, a downstream product of auxin homeostasis, in hydathode-enriched tissue than in leaf blades. This suggested that hydathodes are active sites of auxin synthesis and signalling.
As shown before, the authors found high expression of many genes encoding transporters of water and ions, hormone transporters, and transporters of sugars, peptides, waxes and other organic compounds in hydathodes. Leaf tissues captured 91% of the metabolites before they reached the hydathode. From the pre-hydathode fluid, 78% of the remaining metabolites were captured before the fluid got released by the hydathodes. Metabolites, inorganic phosphate (Pi) and nitrate were taken up by the hydathode, as well as phosphorus, calcium and magnesium, suggesting that hydathodes capture specific organic and mineral compounds before guttation.
The authors focused on nitrate transporter NRT2.1 and the Pi transporter PHT1;4, which are highly expressed in hydathodes and well-studied in roots. Higher nitrate and Pi levels in the guttation fluid of nrt2.1 and pht1;4 mutants suggested that the transporters contribute to the uptake of Pi and nitrate from the guttation fluid. PHT1;4 expression matched Pi accumulation sites in leaves, with more Pi in leaf margins than blades. Metabolites and minerals taken up from the guttation fluid can be recycled back into the leaves: a previous study of a barley leaf fed with 32Pi at hydathodes showed an ingress of Pi from the hydathode after 2 h followed by diffusion to the entire leaf tip after one day. In conclusion, Routaboul et al. speculate that hydathodes act analogously to nephrons in kidneys, filtering the guttation fluid and retrieving valuable nutrients.
Hydathodes are essential for maintaining plant water status and to get rid of excess water that could lead to mesophyll flooding, lower photosynthesis/respiration and leaf necrosis. Beside their role in metabolite scavenging, hydathodes can also mediate excretion of undesired or toxic compounds such as boron or nanoparticles. They are also the main entry for several plant pathogens. Understanding hydathode physiology could thus have implications for adapting plant performance in stressful conditions such as flooding, drought, immunity or phytoremediation.
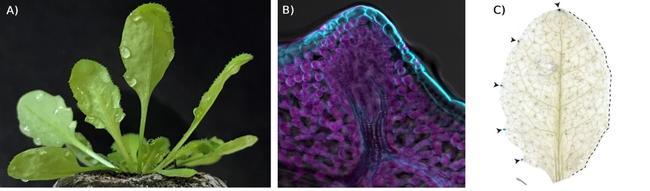
Figure 1: Arabidopsis hydathodes facilitate nutrient recovery from xylem sap.
A) Arabidopsis plant with guttation droplets at hydathodes
B) Confocal image of Arabidopsis hydathode. Overlay of brightfield (grey), autofluorescence (magenta), and staining of cell walls (blue) images.
C) PHT1;4 promoter activity (blue) is detectable at hydathodes, but not at excised margins (Photo credits Jean-Marc Routaboul (A), Yves Martinez and Jean-Marc Routaboul (B) and modified from (Routaboul et al., 2024) (C)).